 All Categories
All Categories
 Intermediate
Intermediate
 400-780-8018
400-780-8018Small molecule compounds typically have a clear chemical structure and biological activity, occupying an important position in the field of drug development. Many drugs are small molecule compounds because they can cross cell membranes and interact with specific biological macromolecules (such as proteins), thereby exerting their therapeutic effects. Crystallization is a routine technique for dealing with organic compounds, and during the crystallization process of small molecule compounds, oiling out phenomena sometimes occur.
The phenomenon of oil precipitation, also known as “oiling,” liquid-liquid demixing, or liquid-liquid phase separation (LLPS), occurs when a solute-enriched phase (the oil phase) separates from the solute-rich aqueous phase (the water phase). The frequency of oil separation in the test depends on the specific test conditions and the material system involved. Oil precipitation may be common under certain substances or reaction conditions, including: low melting point, relatively large relative molecular weight, polypeptides, cyclic lipopeptides; molecular species containing flexible structures, such as long carbon chains that are easy to rotate; and sugars.
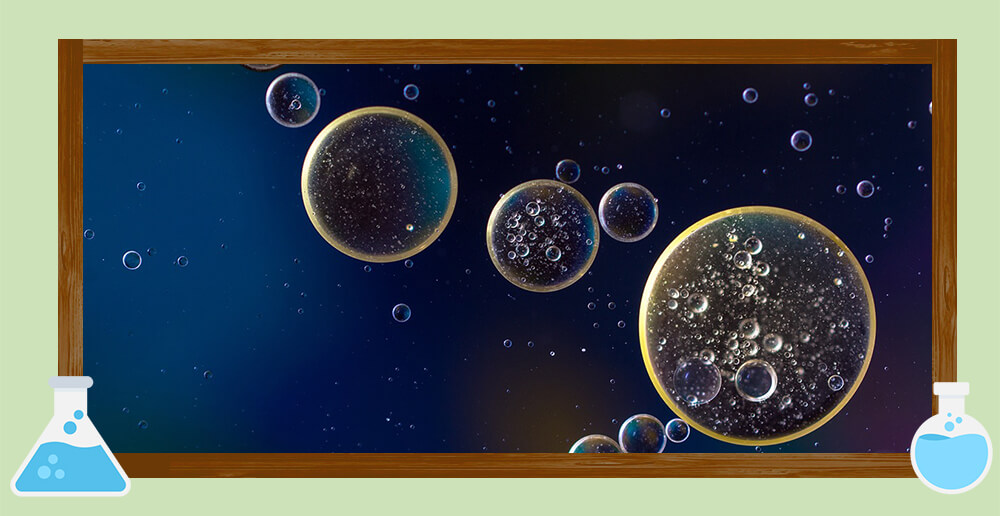
During the test, the phenomenon of oil separation may lead to inaccurate analysis results and affect the normal operation of the test such as the reaction rate slows down, product recovery is difficult, equipment is blocked, and other problems. In order to avoid or solve the phenomenon of oil separation, we can control the supersaturation, add seed crystals, remove impurities, remove solvent residues, control the temperature, replace the solvent system, adjust the pH value, control the operating conditions such as stirring speed to reduce the possibility of oil precipitation.
Slurry conversion is a maturation process where a suspension transitions from a metastable or amorphous state to a more stable crystalline form, as explained by Ostwald’s rule. Continuous stirring is essential to promote uniform distribution and prevent seed crystals from settling in one spot, enabling efficient crystal growth. Both magnetic and mechanical stirring methods are suitable for this process.
If this situation occurs when adding seed crystals, you can choose to increase the amount of seed crystals, or you need to pay attention to the order of adding materials. For example, if the previous crystallization method is to add the antisolvent to the good solvent, then you can consider adding the seed crystal to the poor solvent to form a suspension, and then slowly add the desired crystallized sample dropwise to the solution with the seed crystal. In the solution, be careful to add the material slowly, as this may induce it.

The most reliable method is simulating powder X-ray diffraction (XRPD) diagrams based on single-crystal data. If it is difficult to prepare single crystals of some compounds, you can try multiple batches of tests, such as using different crystallization methods. It turns out that all the XRPD, DSC and TGA data of the sample obtained are consistent, and the XRPD patterns are consistent. At this time, you can basically determine that it is a single crystal form.
The XRPD spectrum of the mixture is the superposition of the XRPD spectra of each component, so the most likely case of multi-peak XRPD is that other crystal forms of the compound are mixed in. Assuming that A and B are mixed crystals, the characteristic peaks of both crystal forms may appear. Because it has fingerprint properties, if you can observe the characteristics of A and B through the XRPD pattern, you can determine that it is a mixed crystal. If the purity of the crystal form is to be determined, quantitative analysis method development is required. It is difficult to tell the proportion of crystal forms just by looking at an XRPD chart.
Use minimal seed crystals to induce the desired crystal form. Repeatedly induce subsequent batches using products from earlier inductions. Over time, this method dilutes and eliminates unwanted crystal forms or impurities.
It looks like a solid, but in fact it is not a real solid (crystal). It is actually a kind of gel. When you suck it up with a disposable straw, you will find that it absorbs moisture immediately and then melts.
Pharmaceutical intermediates are chemical substances critical to the synthesis of Active Pharmaceutical Ingredients (APIs). These intermediate products, including raw materials, precursors, and semi-finished products, form an essential part of the drug synthesis pathway, enabling the transformation into target drug molecules through a series of chemical reactions.
In the synthesis of small molecule compounds, pharmaceutical intermediates play a pivotal role. They are indispensable for enhancing synthesis efficiency, ensuring product quality, and reducing production costs, making them a cornerstone of modern pharmaceutical manufacturing.
Pharmalego specializes in delivering high-quality pharmaceutical intermediates that meet diverse research and development needs. Our products support compound screening for pharmaceutical R&D professionals and provide reliable solutions for drug synthesis. To further assist our clients, we offer end-to-end services, from small-scale customization of pharmaceutical intermediates in early research stages to large-scale production process optimization for late-stage clinical trials. These tailored solutions address the evolving demands of innovative drug development, helping pharmaceutical companies accelerate their drug discovery and commercialization timelines.
 Shanghai ICP Record Number 2023026221-1
Shanghai Public Network Security Registration No.31011502400732
Shanghai ICP Record Number 2023026221-1
Shanghai Public Network Security Registration No.31011502400732